top of page
Resources


What is Temperature Mapping Protocol?
Temperature Mapping protocol helps to detail the steps for the study - "what you plan to do"


What is Temperature Mapping in Manufacturing Industry?
Temperature mapping helps to know and accordingly maintain the temperature variations within a given storage/ warehouse. It is an important audit point for GMP Compliance.


When Should GMP Companies Seek Compliance Consulting to Enhance Audit-Readiness Through Effective Documentation
Meeting Good Manufacturing Practice (GMP) standards is a critical challenge for pharmaceutical and biotech companies. One of the most common hurdles is maintaining thorough and accurate documentation. Without it, audit-readiness suffers, increasing the risk of compliance failures and costly regulatory actions. This post explains when GMP companies should seek GMP consulting, why documentation for GMP Pharma and Biotech audit compliance is essential, and how good documentation


The Rise of Smart Factories: How AI is Powering the Next Generation of Biopharmaceutical Manufacturing
AI influence on Biopharmaceutical Manufacturing


Ensuring Data Integrity Lifecycle Assessment for GMP Compliance
Data Integrity Lifecycle Validation for GMP Compliance

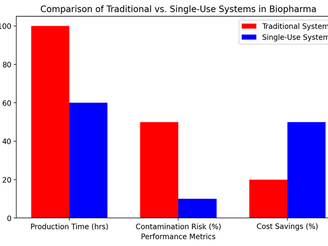
The Evolution and Impact of Single-Use Systems in Biopharma Manufacturing.
Evolution and Impact of Single-Use Systems in Biopharma Manufacturing


Digital Twins in Biopharma: The Future of Process Validation and Manufacturing Optimization
Future of Process Validation and Manufacturing Optimization
bottom of page


